# 1. Dataset information
A set of high-density EEG (electroencephalogram) recording obtained from awake, freely-moving mice (*mus musculus*) (n = 6). Details of experimental method are described in the original research article using the same dataset [Hwang et al., 2019, *Brain Structure and Function*].
* Title: High-density EEG recording in mice for auditory steady-state response with optogenetic stimulation in the basal forebrain
* Authors: Eunjin Hwang, Hio-Been Han, Jeong-Yeong Kim, & Jee Hyun Choi [corresponding: jeechoi@kist.re.kr]
* Version: 1.0.0
* Related publication: [Hwang et al., 2019, *Brain Structure and Function*](https://link.springer.com/article/10.1007/s00429-019-01845-5).
* Dataset repository: G-Node GIN (DOI will be provided soon) https://gin.g-node.org/hiobeen/Mouse_hdEEG_ASSR_Hwang_et_al/
**Step-by-step tutorial is included, fully functioning with _Google Colaboratory_ environment.**
[](https://colab.research.google.com/drive/1LRmR0fxqWFvwxaNIJxMJkMQVblcuTazk)
# 2. File organization
Raw EEG data are saved in EEGLAB dataset format (*.set). Below are the list of files included in this dataset.
**a) Meta data file (1 csv file)**
[metadata.csv]
**b) Electrode montage file (1 csv file)**
[montage.csv]
**c) EEG data files (6 set files, 6 fdt files)**
[rawdata/epochs_animal1.set, rawdata/epochs_animal1.fdt]
[rawdata/epochs_animal2.set, rawdata/epochs_animal2.fdt]
[rawdata/epochs_animal3.set, rawdata/epochs_animal3.fdt]
[rawdata/epochs_animal4.set, rawdata/epochs_animal4.fdt]
[rawdata/epochs_animal5.set, rawdata/epochs_animal5.fdt]
[rawdata/epochs_animal6.set, rawdata/epochs_animal6.fdt]
**d) Example python scripts**
[analysis_tutorial.ipynb]
* written and tested on Google Colab - Python 3 environment
# 3. How to get started (Python 3 without _gin_)
As the data are saved in EEGLAB format, you need to install appropriate module to access the data in Python3 environment. The fastest way would be to use read_epochs_eeglab() function in *MNE-python* module. You can download the toolbox from the link below (or use pip install mne in terminal shell).
*[MNE-python]* https://martinos.org/mne/stable/index.html
## Part 1. Accessing dataset
### 1-1. Download dataset and MNE-python module
The dataset has been uploaded on G-Node and can be accessed by git command, by typing git clone https://gin.g-node.org/hiobeen/Mouse_hdEEG_ASSR_Hwang_et_al. However, it's currently not functioning because of the large size of each dataset (>100 MB). Instead, you can use *gin* command or custom function written below to copy dataset into your work environment. In *gin* repository, a python script download_sample.py is provided. It doesn't require *git* or *gin* command, simply using request module in Python 3. Try typing python download_sample.py on terminal/command after changing desired directory. Demo 1-1 is composed of download_sample.py script in this Jupyter-Notebook document.
> Warning: Direct cloning using *git clone git@gin.g-node.org:/hiobeen/Mouse_hdEEG_ASSR_Hwang_et_al.git* may not work because of the large size of each dataset (>100 MB).
Also, you need to install *MNE-Python* module using *pip* command to load EEGLAB-formatted EEG data. Install command using *pip* is located at the end of script download_sample.py. To download dataset and install MNE-python module into your environment (local machine/COLAB), try running scripts below.
> Note: Through this step-by-step demonstration, we will use data from one animal (#Animal 2). Unnecessary data files will not be downloaded to prevent long download time. To download whole dataset, change dataset_to_download = [2] into dataset_to_download = [1,2,3,4,5,6].
```python
# Demo 1-1. Setting an enviroment (download_sample.py)
from os import listdir, mkdir, path, system, getcwd
import warnings; warnings.simplefilter("ignore")
dir_origin = dir_origin = getcwd()+'/' # <- Change this in local machine
dir_dataset= 'dataset/'
print('\n1)============ Start Downloading =================\n')
print('Target directory ... => [%s%s]'%(dir_origin,dir_dataset))
#!rm -rf /content/dataset/
import requests, time
def download_dataset( dataset_to_download = range(1,7), dir_dataset = dir_dataset ):
# Check directory
if not path.isdir('%s%s'%(dir_origin,dir_dataset)):
mkdir('%s%s'%(dir_origin,dir_dataset))
mkdir('%s%s/rawdata/'%(dir_origin,dir_dataset))
# File names to be downloaded
file_ids = [ 'meta.csv', 'montage.csv' ]
for set_id in dataset_to_download:
file_ids.append( 'rawdata/epochs_animal%s.set'%set_id )
file_ids.append( 'rawdata/epochs_animal%s.fdt'%set_id )
# Request & download
repo_url = 'https://gin.g-node.org/hiobeen/Mouse_hdEEG_ASSR_Hwang_et_al/raw/3fa95eeb11021cf740ded020c9948c6aa0d1c904/'
for file_id in file_ids:
fname_dest = "%s%s%s"%(dir_origin, dir_dataset, file_id)
if path.isfile(fname_dest) is False:
print('...copying to [%s]...'%fname_dest)
file_url = '%s%s'%(repo_url, file_id)
r = requests.get(file_url, stream = True)
with open(fname_dest, "wb") as file:
for block in r.iter_content(chunk_size=1024):
if block: file.write(block)
time.sleep(1) # wait a second to prevent possible errors
else:
print('...skipping already existing file [%s]...'%fname_dest)
# Initiate downloading
dataset_to_download = [2] # Partial download to prevent long download time
#dataset_to_download = [1,2,3,4,5,6] # Full download
download_dataset(dataset_to_download)
print('\n============= Download finished ==================\n\n')
# List up 'dataset/' directory
print('\n2)=== List of available files in google drive ====\n')
print(listdir('%sdataset/'%dir_origin))
print('\n============= End of the list ==================\n\n')
# List up dataset/rawdata/*.set and ~/*.fdt files
print('\n3)=== List of available files in google drive ====\n')
print(listdir('%sdataset/rawdata/'%dir_origin))
print('\n============= End of the list ==================\n\n')
# Install mne-python module
system('pip install mne');
# Make figure output directory
dir_fig = 'figures/'
if not path.isdir(dir_fig): mkdir('%s%s'%(dir_origin, dir_fig))
```
1)============ Start Downloading =================
Target directory ... => [/content/dataset/]
...copying to [/content/dataset/meta.csv]...
...copying to [/content/dataset/montage.csv]...
...copying to [/content/dataset/rawdata/epochs_animal2.set]...
...copying to [/content/dataset/rawdata/epochs_animal2.fdt]...
============= Download finished ==================
2)=== List of available files in google drive ====
['meta.csv', 'montage.csv', 'rawdata']
============= End of the list ==================
3)=== List of available files in google drive ====
['epochs_animal2.set', 'epochs_animal2.fdt']
============= End of the list ==================
### 1-2. Accessing meta-data table
File *meta.csv* contains the demographic information of 6 mice. Using read_csv() of *pandas* module, meta-datat able can be visualized as follow.
```python
## Demo 1-2. Display meta-data file
from pandas import read_csv
meta = read_csv('%s%smeta.csv'%(dir_origin, dir_dataset));
print('Table 1. Meta-data')
meta
```
Table 1. Meta-data
|
subject_name |
file_name |
n_trials |
age_in_month |
sex |
| 0 |
animal1 |
epochs_animal1.set |
589 |
4 |
Male |
| 1 |
animal2 |
epochs_animal2.set |
557 |
4 |
Male |
| 2 |
animal3 |
epochs_animal3.set |
349 |
4 |
Male |
| 3 |
animal4 |
epochs_animal4.set |
493 |
4 |
Female |
| 4 |
animal5 |
epochs_animal5.set |
956 |
5 |
Male |
| 5 |
animal6 |
epochs_animal6.set |
959 |
4 |
Male |
```python
# (Optional: Check total number of trials for each experimental condition)
"""
# Count number of trials for each experimental condition (all files)
import pandas as pd
n_trials = np.zeros((7,8),dtype='int')
for dataset_idx in range(6):
EEG_temp, f_name = get_eeg_data( dataset_idx )
for condition in range(1,8):
# Accessing event info: print( EEG.event )
trialIdx = np.where(EEG_temp.events[:,2]==condition)[0]
n_trials[dataset_idx,condition-1] = len(trialIdx)
n_trials[-1,:], n_trials[:,-1] = np.sum(n_trials,axis=0), np.sum(n_trials,axis=1)
n_trials = pd.DataFrame(n_trials, index=['animal1','animal2','animal3','animal4','animal5','animal6', 'Total'],
columns=['In-phase','Out-of-phase','Delayed','Advanced','Continuous','Sound only', 'Light only', 'Total'])
n_trials
""";
```
### 1-3. Data loading and dimensionality check
Each _*.fdt_ file is consisted of different number of trials. To load dataset, a function get_eeg_data() is defined below. To maintain original dimensionality order (cf. channel-time-trial in EEGLAB of Matlab), np.moveaxis() was applied.
```python
# Demo 1-3. Data loading and dimensionality check
from mne.io import read_epochs_eeglab as loadeeg
import numpy as np
def get_eeg_data(dataset_idx=1, CAL=1e-6):
f_name = '%s%srawdata/%s'%(dir_origin,dir_dataset,meta.file_name[dataset_idx])
EEG = loadeeg(f_name, verbose=False)
EEG.data = np.moveaxis(EEG.get_data(), 0, 2) / CAL
return EEG, f_name
# Data loading
EEG, f_name = get_eeg_data( dataset_idx = 1 ) # idx = from 0 to 5 (1st to 6th)
# Dimension check
print('File name : [%s]'%f_name)
print('File contains [%d channels, %4d time points, %3d trials]'%(EEG.data.shape))
```
File name : [/content/dataset/rawdata/epochs_animal2.set]
File contains [38 channels, 5200 time points, 557 trials]
Note that voltage calibration value (*CAL*) is set to 1e-6 in 0.11.0 version of [eeglab.py](https://github.com/mne-tools/mne-python/blob/master/mne/io/eeglab/eeglab.py]).
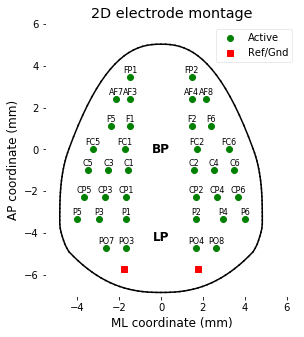
### 1-4. Getting channel coordinates
The EEG data are recorded with 38 electrode array, and two of the electrodes were used as ground and reference site - total 36 channel data are available. Coordinates of each electrode are in the file [data/montage.csv], and can be accessed and visualized by following script.
```python
# Demo 1-4. Import montage matrix
from matplotlib import pyplot as plt; plt.style.use('ggplot')
plt.rcParams['font.family']='sans-serif'
plt.rcParams['text.color']='black'; plt.rcParams['axes.labelcolor']='black'
plt.rcParams['xtick.color']='black'; plt.rcParams['ytick.color']='black'
from pandas import read_csv
montage_table = read_csv('%s%smontage.csv'%(dir_origin, dir_dataset))
elec_montage = np.array(montage_table)[:, 1:3]
# Open figure handle
plt.figure(figsize=(4.5,5))
# Plot EEG channels position (total 36 channels)
plt.plot( elec_montage[:36,0], elec_montage[:36,1], 'go' )
for chanIdx in range(36):
plt.text( elec_montage[chanIdx,0], elec_montage[chanIdx,1]+.2,
EEG.info['ch_names'][chanIdx][5:], ha='center', fontsize=8 )
# Plot Ref/Gnd electrode position
plt.plot( elec_montage[36:,0], elec_montage[36:,1], 'rs' )
plt.text(0, 0.0, 'BP', fontsize=12, weight='bold', ha='center',va='center');
plt.text(0,-4.2, 'LP', fontsize=12, weight='bold', ha='center',va='center');
plt.xlabel('ML coordinate (mm)'); plt.ylabel('AP coordinate (mm)');
plt.title('2D electrode montage');
plt.legend(['Active','Ref/Gnd'], loc='upper right', facecolor='w');
plt.gca().set_facecolor((1,1,1))
plt.grid(False); plt.axis([-5.5, 6.5, -7, 6])
# Draw head boundary
def get_boundary():
return np.array([
-4.400, 0.030, -4.180, 0.609, -3.960, 1.148, -3.740, 1.646, -3.520, 2.105, -3.300, 2.525, -3.080, 2.908, -2.860, 3.255,
-2.640, 3.566, -2.420, 3.843, -2.200, 4.086, -1.980, 4.298, -1.760, 4.4799, -1.540, 4.6321, -1.320, 4.7567, -1.100, 4.8553,
-0.880, 4.9298, -0.660, 4.9822, -0.440, 5.0150, -0.220, 5.0312,0, 5.035, 0.220, 5.0312, 0.440, 5.0150, 0.660, 4.9822,
0.880, 4.9298, 1.100, 4.8553, 1.320, 4.7567, 1.540, 4.6321,1.760, 4.4799, 1.980, 4.2986, 2.200, 4.0867, 2.420, 3.8430,
2.640, 3.5662, 2.860, 3.2551, 3.080, 2.9087, 3.300, 2.5258,3.520, 2.1054, 3.740, 1.6466, 3.960, 1.1484, 4.180, 0.6099,
4.400, 0.0302, 4.400, 0.0302, 4.467, -0.1597, 4.5268, -0.3497,4.5799, -0.5397, 4.6266, -0.7297, 4.6673, -0.9197, 4.7025, -1.1097,
4.7326, -1.2997, 4.7579, -1.4897, 4.7789, -1.6797, 4.7960, -1.8697,4.8095, -2.0597, 4.8199, -2.2497, 4.8277, -2.4397, 4.8331, -2.6297,
4.8366, -2.8197, 4.8387, -3.0097, 4.8396, -3.1997, 4.8399, -3.3897,4.8384, -3.5797, 4.8177, -3.7697, 4.7776, -3.9597, 4.7237, -4.1497,
4.6620, -4.3397, 4.5958, -4.5297, 4.5021, -4.7197, 4.400, -4.8937,4.1800, -5.1191, 3.9600, -5.3285, 3.7400, -5.5223, 3.5200, -5.7007,
3.3000, -5.8642, 3.0800, -6.0131, 2.8600, -6.1478, 2.6400, -6.2688,2.4200, -6.3764, 2.2000, -6.4712, 1.9800, -6.5536, 1.7600, -6.6241,
1.5400, -6.6833, 1.3200, -6.7317, 1.1000, -6.7701, 0.8800, -6.7991,0.6600, -6.8194, 0.4400, -6.8322, 0.2200, -6.8385, 0, -6.840,
-0.220, -6.8385, -0.440, -6.8322, -0.660, -6.8194, -0.880, -6.7991,-1.100, -6.7701, -1.320, -6.7317, -1.540, -6.6833, -1.760, -6.6241,
-1.980, -6.5536, -2.200, -6.4712, -2.420, -6.3764, -2.640, -6.2688,-2.860, -6.1478, -3.080, -6.0131, -3.300, -5.8642, -3.520, -5.7007,
-3.740, -5.5223, -3.960, -5.3285, -4.180, -5.1191, -4.400, -4.89370,-4.5021, -4.7197, -4.5958, -4.5297, -4.6620, -4.3397, -4.7237, -4.1497,
-4.7776, -3.9597, -4.8177, -3.7697, -4.8384, -3.5797, -4.8399, -3.3897,-4.8397, -3.1997, -4.8387, -3.0097, -4.8367, -2.8197, -4.8331, -2.6297,
-4.8277, -2.4397, -4.8200, -2.2497, -4.8095, -2.0597, -4.7960, -1.8697,-4.7789, -1.6797, -4.7579, -1.4897, -4.7326, -1.2997, -4.7025, -1.1097,
-4.6673, -0.9197, -4.6266, -0.7297, -4.5799, -0.5397, -4.5268, -0.3497,-4.4670, -0.1597, -4.4000, 0.03025]).reshape(-1, 2)
boundary = get_boundary()
for p in range(len(boundary)-1): plt.plot(boundary[p:p+2,0],boundary[p:p+2,1], 'k-')
#plt.axis((-5))
plt.gcf().savefig(dir_fig+'fig1-4.png', format='png', dpi=300);
```
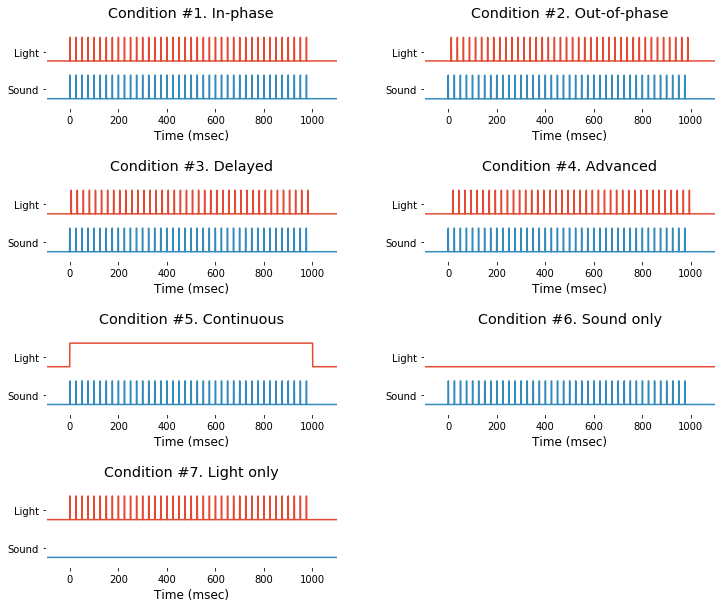
## Part 2. Plotting Event-Related Potentials
### 2-1. Accessing event info
Event information is saved in struct-type variable EEG.event and you can access it by typing EEG.event. Also, their time trace are avilable in 37-th and 38-th channel of EEG.data. For demonstration purpose, light and sound stimuli of 7 different types of event can be extracted and drawn as follow.
```python
# Demo 2-1. Event profile (sound/light stimuli)
condNames = ['In-phase','Out-of-phase','Delayed','Advanced',
'Continuous','Sound only', 'Light only'];
plt.figure(figsize=(12,10))
yshift = .8;
for condition in range(1,len(condNames)+1):
plt.subplot(4,2,condition)
trialIdx = np.where(EEG.events[:,2]==condition)[0]
# Light stim
light = EEG.data[-2,:,trialIdx[0]] + yshift
plt.plot( EEG.times*1000, light)
# Sound stim
sound = EEG.data[-1,:,trialIdx[0]] - yshift
plt.plot( EEG.times*1000, sound)
plt.ylim([-1.5*yshift, 3*yshift])
plt.xlim([-.10*1000, 1.10*1000])
plt.xlabel('Time (msec)')
plt.yticks( (yshift*-.5,yshift*1.5), labels=['Sound', 'Light'] )
plt.title('Condition #%d. %s'%(condition,condNames[condition-1]))
plt.gca().set_facecolor((1,1,1))
plt.subplots_adjust(wspace=.3, hspace=.8)
plt.gcf().savefig(dir_fig+'fig2-1.png', format='png', dpi=300);
```
### 2-2. Visualizing example single-trial trace
If data is successfully loaded, now you're ready! For data visualization, an example function is provided below.
The function plot_multichan() draws multi-channel time series data, by taking 1D time vector, x, and 2D data matrix, y.
```python
# Demo 2-2a. Function for multi-channel plotting
def plot_multichan( x, y, spacing = 3000, figsize = (10,10), ch_names = EEG.ch_names ):
# Set color theme
color_template = np.array([[1,.09,.15],[1,.75,.28],[.4,.2,0],[.6,.7,.3],[.55,.55,.08]])
color_space = np.tile( color_template,
(int(np.ceil([ float(y.shape[0])/color_template.shape[0]])[0]), 1) )
# Open figure and plot
plt.figure(figsize=figsize)
y_center = np.linspace( -spacing, spacing, y.shape[0] )
for chanIdx in range(y.shape[0]):
shift = y_center[chanIdx] + np.nanmean(y[chanIdx,:])
plt.plot(x, y[chanIdx,:]-shift, color=color_space[chanIdx,], linewidth=1);
plt.xlabel('Time (sec)')
plt.ylim((-1.1*spacing,1.1*spacing))
plt.yticks(y_center, ch_names[::-1]);
plt.gca().set_facecolor((1,1,1))
return y_center
```
Using plot_multichan() function, example single-trial EEG trace can be visualized as follow.
```python
# Demo 2-2b. Visualization of raw EEG time trace
trial_index = 0
y_center = plot_multichan(EEG.times, EEG.data[:,:,trial_index])
plt.title('Example trial (index=%d) trace'%(1+trial_index));
plt.gcf().savefig(dir_fig+'fig2-2.png', format='png', dpi=300);
```

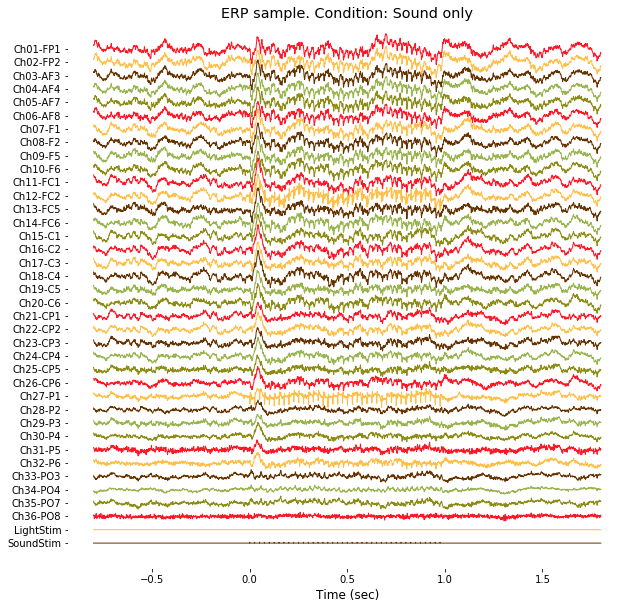
Note that channels 1 to 36 contain actual EEG data from 36-channel electrode array (from FP1 to PO8), and channel 37 and 38 contain binary stimulus profile (0: no stimulation, 1: stimulation) of light and sound, respectively.
### 2-3. ERP in time domain
Using same function, plot_multichan(), ERP (Event-related potentials) trace can be drawn as follow.
```python
# Demo 2-3. Visualization of ERP time trace
condNames = ['In-phase', 'Out-of-phase', 'Delayed', 'Advanced',
'Continuous','Sound only', 'Light only']
targetCondition = 6 # <- Try changing this
trialIdx = np.where((EEG.events[:,2])==targetCondition)[0]
erp = np.nanmean(EEG.data[:,:,trialIdx],2)
c = plot_multichan(EEG.times, erp, spacing = 300 )
plt.title('ERP sample. Condition: %s'%(condNames[targetCondition-1]));
plt.gcf().savefig(dir_fig+'fig2-3.png', format='png', dpi=300);
```
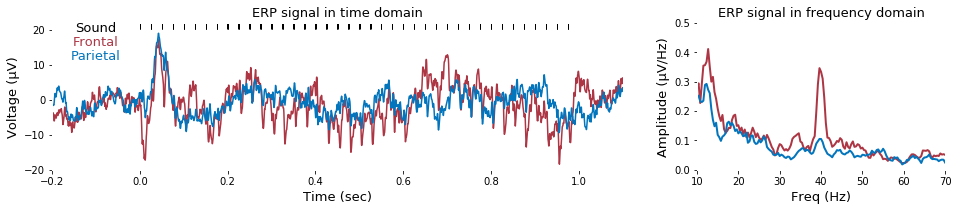
### 2-4. ERP in frequency domain
To calculate the amplitude of 40-Hz auditory steady-state response, fast Fourier transform can be applied as follow.
```python
# Demo 2-4. Time- and frequency-domain visualization of grand-averaged ERP
def fft_half(x, Fs=2000): return np.fft.fft(x)[:int(len(x)/2)]/len(x), np.linspace(0,Fs/2,len(x)/2)
plt.figure(figsize=(16,3))
trialIdx = np.where((EEG.events[:,2])==6)[0]
# Channel selection
ch_frontal = (2,6) # channel index of frontal electrodes
ch_parietal = (20,24) # channel index of parietal electrodes
print( 'Channel selection\n..Frontal channels -> %s'%EEG.info['ch_names'][ch_frontal[0]:ch_frontal[1]] )
print( '..Parietal channels -> %s\n'%EEG.info['ch_names'][ch_parietal[0]:ch_parietal[1]] )
# Calc ERP traces for visualization
erp = np.mean(EEG.data[:,:,trialIdx],2)
frontal_erp = np.mean(erp[ch_frontal[0]:ch_frontal[1],:],0) # Average of frontal-area channels
parietal_erp = np.mean(erp[ch_parietal[0]:ch_parietal[1],:],0) # Average of parietal-area channels
frontal_erp_fft,freq = fft_half(frontal_erp)
parietal_erp_fft,freq = fft_half(parietal_erp)
sound_stim = np.where(erp[-1,:])[0]
# Plot ERP (Time)
font_size = 13
color_f = (.68,.210,.27) # Custom color value
color_p = (.01,.457,.74)
plt.subplot(1,3,(1,2))
plt.grid('off')
plt.plot( EEG.times, frontal_erp, color= color_f)
plt.plot( EEG.times, parietal_erp, color= color_p)
plt.xlim((-.2,1.1))
plt.ylim((-20,22))
plt.text( -.1, 19.5, 'Sound', ha='center', fontsize=font_size, color='k' )
plt.text( -.1, 15.5, 'Frontal', ha='center', fontsize=font_size, color=color_f )
plt.text( -.1, 11.5,'Parietal', ha='center', fontsize=font_size, color=color_p )
plt.title('ERP signal in time domain', fontsize=font_size)
plt.xlabel('Time (sec)', fontsize=font_size)
plt.ylabel('Voltage (μV)', fontsize=font_size)
plt.plot( EEG.times[sound_stim], np.zeros(sound_stim.shape)+21, 'k|' )
plt.gca().set_facecolor((1,1,1))
# Plot ERP (Frequency)
def smoothing(x, L=5): # smoothing function
return np.convolve(np.ones(L,'d')/np.ones(L,'d').sum(),
np.r_[x[L-1:0:-1],x, x[-2:-L-1:-1]],mode='valid')[round((L-1)*.5):round(-(L-1)*.5)]
plt.subplot(1,3,3)
plt.plot( freq, smoothing(np.abs(frontal_erp_fft)), linewidth=2, color=color_f )
plt.plot( freq, smoothing(np.abs(parietal_erp_fft)), linewidth=2, color=color_p )
plt.xlim((10,70))
plt.ylim((0,.5))
plt.xlabel('Freq (Hz)', fontsize=font_size)
plt.ylabel('Amplitude (μV/Hz)', fontsize=font_size)
plt.title('ERP signal in frequency domain', fontsize=font_size)
plt.gca().set_facecolor((1,1,1))
plt.subplots_adjust(wspace=.3, hspace=.1, bottom=.2)
plt.gcf().savefig(dir_fig+'fig2-4.png', format='png', dpi=300);
```
Channel selection
..Frontal channels -> ['Ch03-AF3', 'Ch04-AF4', 'Ch05-AF7', 'Ch06-AF8']
..Parietal channels -> ['Ch21-CP1', 'Ch22-CP2', 'Ch23-CP3', 'Ch24-CP4']
### 2-5. ERP in time-frequency domain
Applying fast Fourier transform with moving temporal window, ERP signal can be drawn in time-frequency domain. To calculate spectrogram, a function get_spectrogram() is defined.
```python
# Demo 2-5. Visualize frequency components in ERP
def get_spectrogram( data, t=EEG.times, Fs=2000,
fft_win_size=2**10, t_resolution=0.1, freq_cut = 150):
# For many- and single-trials data compatibility
if data.ndim < 3: data = np.expand_dims(data,2)
t_fft = [t[0]+(((fft_win_size*.5)+1)/Fs),
t[-1]-(((fft_win_size*.5)+1)/Fs)];
t_vec = np.linspace( t_fft[0], t_fft[-1], (np.diff(t_fft)/t_resolution)+1);
# Memory pre-occupation
n_ch, _, n_trial = data.shape
n_t = len(t_vec);
_,f = fft_half( np.zeros(fft_win_size), Fs);
n_f = np.where(f<100)[0][-1]+1;
Spec = np.zeros( [n_t, n_f, n_ch, n_trial], dtype='float16');
Spec_f = f[0:n_f];
# Get sliding window indicies
idx_collection = np.zeros((len(t_vec),2), dtype='int')
for tIdx in range(len(t_vec)):
idx_collection[tIdx,0] = int(np.where(tplot_topo2d( data ), is provided which takes data as 1D data matrix (i.e., 1 x 36 channels) each of which represents the channel power.
For 2D interpolation, additional *class* of bi_interp2 is defined.
```python
# Demo 3-1a. Preparation of 2D power topography
""" (1) Class for 2D interpolation """
import numpy as np
import matplotlib.pyplot as plt
from scipy import interpolate
class bi_interp2:
def __init__(self, x, y, z, xb, yb, xi, yi, method='linear'):
self.x = x
self.y = y
self.z = z
self.xb = xb
self.yb = yb
self.xi = xi
self.yi = yi
self.x_new, self.y_new = np.meshgrid(xi, yi)
self.id_out = np.zeros([len(self.xi), len(self.xi)], dtype='bool')
self.x_up, self.y_up, self.x_dn, self.y_dn = [], [], [], []
self.interp_method = method
self.z_new = []
def __call__(self):
self.find_boundary()
self.interp2d()
return self.x_new, self.y_new, self.z_new
def find_boundary(self):
self.divide_plane()
# sort x value
idup = self.sort_arr(self.x_up)
iddn = self.sort_arr(self.x_dn)
self.x_up = self.x_up[idup]
self.y_up = self.y_up[idup]
self.x_dn = self.x_dn[iddn]
self.y_dn = self.y_dn[iddn]
self.remove_overlap()
# find outline, use monotone cubic interpolation
ybnew_up = self.interp1d(self.x_up, self.y_up, self.xi)
ybnew_dn = self.interp1d(self.x_dn, self.y_dn, self.xi)
for i in range(len(self.xi)):
idt1 = self.y_new[:, i] > ybnew_up[i]
idt2 = self.y_new[:, i] < ybnew_dn[i]
self.id_out[idt1, i] = True
self.id_out[idt2, i] = True
# expand data points
self.x = np.concatenate((self.x, self.x_new[self.id_out].flatten(), self.xb))
self.y = np.concatenate((self.y, self.y_new[self.id_out].flatten(), self.yb))
self.z = np.concatenate((self.z, np.zeros(np.sum(self.id_out) + len(self.xb))))
def interp2d(self):
pts = np.concatenate((self.x.reshape([-1, 1]), self.y.reshape([-1, 1])), axis=1)
self.z_new = interpolate.griddata(pts, self.z, (self.x_new, self.y_new), method=self.interp_method)
self.z_new[self.id_out] = np.nan
def remove_overlap(self):
id1 = self.find_val(np.diff(self.x_up) == 0, None)
id2 = self.find_val(np.diff(self.x_dn) == 0, None)
for i in id1:
temp = (self.y_up[i] + self.y_up[i+1]) / 2
self.y_up[i+1] = temp
self.x_up = np.delete(self.x_up, i)
self.y_up = np.delete(self.y_up, i)
for i in id2:
temp = (self.y_dn[i] + self.y_dn[i + 1]) / 2
self.y_dn[i+1] = temp
self.x_dn = np.delete(self.x_dn, i)
self.y_dn = np.delete(self.y_dn, i)
def divide_plane(self):
ix1 = self.find_val(self.xb == min(self.xb), 1)
ix2 = self.find_val(self.xb == max(self.xb), 1)
iy1 = self.find_val(self.yb == min(self.yb), 1)
iy2 = self.find_val(self.yb == max(self.yb), 1)
# divide the plane with Quadrant
qd = np.zeros([self.xb.shape[0], 4], dtype='bool')
qd[:, 0] = (self.xb > self.xb[iy2]) & (self.yb > self.yb[ix2])
qd[:, 1] = (self.xb > self.xb[iy1]) & (self.yb < self.yb[ix2])
qd[:, 2] = (self.xb < self.xb[iy1]) & (self.yb < self.yb[ix1])
qd[:, 3] = (self.xb < self.yb[iy2]) & (self.yb > self.yb[ix1])
# divide the array with y axis
self.x_up = self.xb[qd[:, 0] | qd[:, 3]]
self.y_up = self.yb[qd[:, 0] | qd[:, 3]]
self.x_dn = self.xb[qd[:, 1] | qd[:, 2]]
self.y_dn = self.yb[qd[:, 1] | qd[:, 2]]
def find_val(self, condition, num_of_returns):
# find the value that satisfy the condition
ind = np.where(condition == 1)
return ind[:num_of_returns]
def sort_arr(self, arr):
# return sorting index
return sorted(range(len(arr)), key=lambda i: arr[i])
def interp1d(self, xx, yy, xxi):
# find the boundary line
interp_obj = interpolate.PchipInterpolator(xx, yy)
return interp_obj(xxi)
""" (2) Function for Topography plot """
from pandas import read_csv
from matplotlib import cm
from mpl_toolkits.mplot3d import Axes3D
def plot_topo2d(data, clim=(-15,25), montage_file='%s%smontage.csv'%(dir_origin, dir_dataset), plot_opt = True):
# Zero-padding
short = 38-len(data)
if short: data=np.concatenate((data, np.tile(.00000001, short)), axis=0)
# Get head boundary image coordinates
boundary = get_boundary()
montage_table = read_csv(montage_file)
x, y = np.array(montage_table['X_ML']), np.array(montage_table['Y_AP'])
xb, yb = boundary[:, 0], boundary[:, 1]
xi, yi = np.linspace(min(xb), max(xb), 500),np.linspace(min(yb), max(yb), 500)
xx, yy, topo_data = bi_interp2(x, y, data, xb, yb, xi, yi)()
if plot_opt:
topo_to_draw = topo_data.copy()
topo_to_draw[np.where(topo_data>clim[1])] = clim[1]
topo_to_draw[np.where(topo_data .001:
bad_channels.append(chIdx)
marker = '> '
else: marker = ' '
if pval > 1: pval=1
print( marker+'Ch=%02d) p = %.5f, R_mean = %.3f, R_std = %.3f'%(
chIdx+1, pval, np.mean(r_data), np.std(r_data)))
print('\nLow-correlated (bad) channels: %s'%(bad_channels))
```
Ch=01) p = 0.00000, R_mean = 0.561, R_std = 0.335
Ch=02) p = 0.00000, R_mean = 0.537, R_std = 0.340
Ch=03) p = 0.00000, R_mean = 0.679, R_std = 0.301
Ch=04) p = 0.00000, R_mean = 0.657, R_std = 0.324
Ch=05) p = 0.00000, R_mean = 0.651, R_std = 0.321
Ch=06) p = 0.00000, R_mean = 0.618, R_std = 0.338
Ch=07) p = 0.00000, R_mean = 0.742, R_std = 0.268
Ch=08) p = 0.00000, R_mean = 0.736, R_std = 0.289
Ch=09) p = 0.00000, R_mean = 0.744, R_std = 0.272
Ch=10) p = 0.00000, R_mean = 0.711, R_std = 0.301
Ch=11) p = 0.00000, R_mean = 0.762, R_std = 0.223
Ch=12) p = 0.00000, R_mean = 0.711, R_std = 0.231
Ch=13) p = 0.00000, R_mean = 0.763, R_std = 0.246
Ch=14) p = 0.00000, R_mean = 0.715, R_std = 0.282
Ch=15) p = 0.00000, R_mean = 0.747, R_std = 0.207
Ch=16) p = 0.00000, R_mean = 0.755, R_std = 0.224
Ch=17) p = 0.00000, R_mean = 0.753, R_std = 0.214
Ch=18) p = 0.00000, R_mean = 0.749, R_std = 0.232
Ch=19) p = 0.00000, R_mean = 0.765, R_std = 0.223
Ch=20) p = 0.00000, R_mean = 0.721, R_std = 0.270
Ch=21) p = 0.00000, R_mean = 0.701, R_std = 0.193
Ch=22) p = 0.00000, R_mean = 0.733, R_std = 0.202
Ch=23) p = 0.00000, R_mean = 0.724, R_std = 0.194
Ch=24) p = 0.00000, R_mean = 0.769, R_std = 0.223
Ch=25) p = 0.00000, R_mean = 0.752, R_std = 0.181
Ch=26) p = 0.00000, R_mean = 0.710, R_std = 0.257
Ch=27) p = 0.00000, R_mean = 0.398, R_std = 0.163
Ch=28) p = 0.00000, R_mean = 0.648, R_std = 0.182
Ch=29) p = 0.00000, R_mean = 0.647, R_std = 0.188
Ch=30) p = 0.00000, R_mean = 0.737, R_std = 0.197
Ch=31) p = 0.00000, R_mean = 0.619, R_std = 0.140
Ch=32) p = 0.00000, R_mean = 0.658, R_std = 0.228
> Ch=33) p = 0.05145, R_mean = 0.106, R_std = 0.306
Ch=34) p = 0.00000, R_mean = 0.262, R_std = 0.135
> Ch=35) p = 0.01168, R_mean = 0.124, R_std = 0.270
Ch=36) p = 0.00000, R_mean = 0.281, R_std = 0.123
Low-correlated (bad) channels: [32, 34]
### 3-2. Time-course of raw voltage topography
Input data of EEG topography can be defined by any mean; voltage, band-limited power, instantaneous angle, and so on. In this example, spatial distribution of raw voltage at specific time point is drawn. For better understanding of the data, ERP time traces at frontal and parietal area are also drawn.
```python
# Demo 3-2. Raw voltage topography
clim = [-20,30]
for targetCondition in conditions:
trialIdx = np.where((EEG.events[:,2])==targetCondition)[0]
# Calc ERP traces for visualization
erp = np.mean(EEG.data[:,:,trialIdx],2)
frontal_erp = np.mean(erp[ch_frontal[0]:ch_frontal[1],:],0) # Average of frontal-area channels
parietal_erp = np.mean(erp[ch_parietal[0]:ch_parietal[1],:],0) # Average of parietal-area channels
# Plot ERP trace
plt.figure(figsize=(14,20))
plt.subplot(len(conditions),1,np.where(np.array(conditions)==targetCondition)[0]+1)
color_f = (.68,.210,.27) # Custom color value
color_p = (.01,.457,.74)
plt.grid('off')
plt.plot((0,0),(-30,45), '--', linewidth = 1, color = (.8,.8,.8))
plt.plot((-.2,1), (0,0), '--', linewidth = 1, color = (.8,.8,.8))
plt.plot( EEG.times, frontal_erp, color= color_f)
plt.plot( EEG.times, parietal_erp, color= color_p)
plt.xlabel('Time (msec)')
plt.xlim((-.2,1))
plt.ylim((-30,45))
plt.axis('off')
plt.gca().set_facecolor((1,1,1))
plt.text( -.1, 27, 'Frontal', ha='center', fontsize=12, color=color_f )
plt.text( -.1, 20,'Parietal', ha='center', fontsize=12, color=color_p )
plt.title('%s'%condNames[targetCondition-1])
# Calculate topography data
t_slice = [ (.005, .025), (.0250, .0550), (.200, .210),(.502, .512), (.925, .935) ]
y_mark = [-20, 33, 10, 5, 10]
topos = []
for tIdx in range(len(t_slice)):
x_start, x_end, y_pos = t_slice[tIdx][0], t_slice[tIdx][1], y_mark[tIdx]
idx_start, idx_end = np.where(EEG.times==x_start)[0][0], np.where(EEG.times==x_end)[0][0]
plt.plot( EEG.times[[idx_start,idx_end]], [y_pos, y_pos], 'k|-')
topo_in = np.mean( erp[:36,idx_start:idx_end],1 )
# bad-channel replacement
topo_in[bad_channels] = np.median( topo_in.flatten() )
topos.append( plot_topo2d(topo_in, plot_opt = False)[2] ) # Save it for drawing
# Draw topography on ERP trace
topo_size = (.07, 21) # X, Y size of topo image
topo_shift = [ (-.04, -16), (.10, 32), (.20, 25), (.512, 20), (.935, 25) ]
topo_clim = np.linspace(clim[0],clim[1],200)
for tIdx in range(len(t_slice)):
topo_x = np.linspace(topo_shift[tIdx][0]-topo_size[0]*.5,topo_shift[tIdx][0]+topo_size[0]*.5,topos[tIdx].shape[0])
topo_y = np.linspace(topo_shift[tIdx][1]-topo_size[1]*.5,topo_shift[tIdx][1]+topo_size[1]*.5,topos[tIdx].shape[1])
plt.contourf( topo_x, topo_y, topos[tIdx], cmap=cm.jet, levels=topo_clim )
plt.draw()
plt.gcf().savefig(dir_fig+'fig3-2.png', format='png', dpi=300);
```
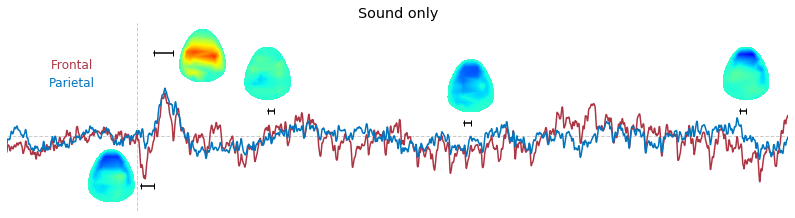
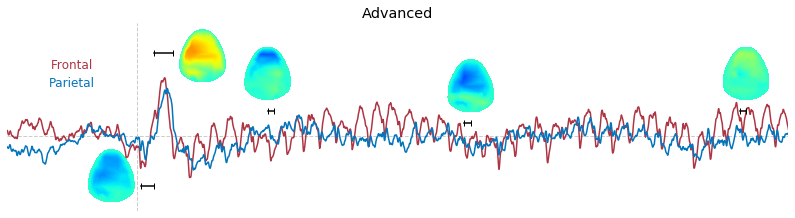
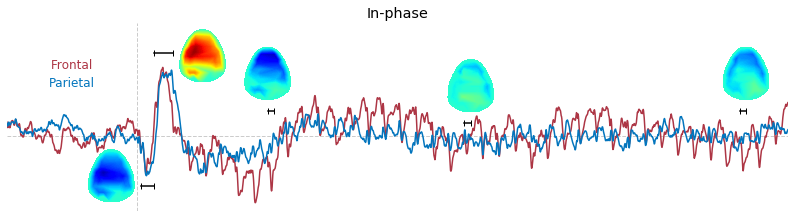
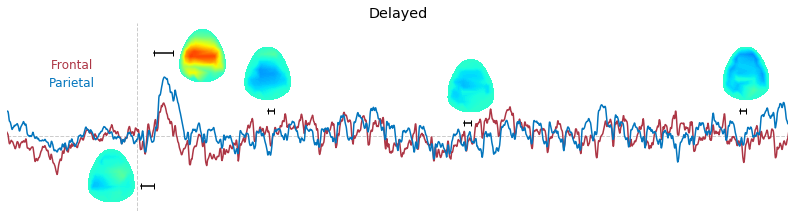
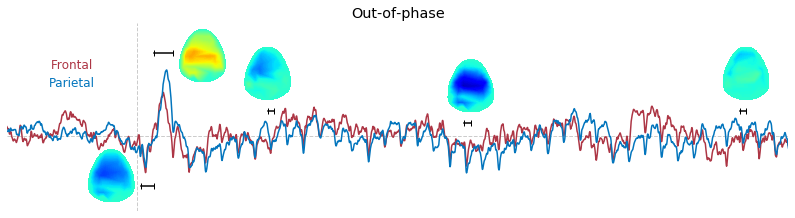
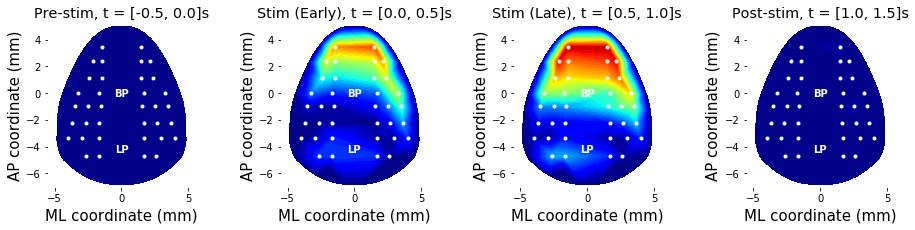
### 3-3. Band-limited power topography
Other than raw voltage, topography of band-limited power at stimulation frequency (40 Hz) can be drawn as well. In this example, stimulus-evoked 40 Hz power were estimated using bandpower() function.
To demonstrate the effect of stimulation, stimulus-free periods (e.g., pre- and post-stimulus period) data are also obtained.
```python
# Demo 3-3. Band-power topography: ERP response as a function of time and space
def band_power(x, targetBand, Fs=2000):
if x.ndim==1:
X, freq = fft_half(x,Fs)
ind = np.where( (freq > targetBand[0]) & (freq <= targetBand[1]))
power = np.sum( abs(X[ind])**2 )
else:
power = np.zeros( x.shape[0] )
for ch in range(x.shape[0]):
X,freq = fft_half(x[ch,],Fs)
ind = np.where( (freq > targetBand[0]) & (freq <= targetBand[1]))
power[ch]=np.sum( abs(X[ind])**2 )
return power
targetCondition = 6 # = Auditory sound only
trialIdx = np.where((EEG.events[:,2])==targetCondition)[0]
erp = np.mean(EEG.data[:,:,trialIdx],2)
period = [ (-.5,0.), (0.,.5), (.5, 1.), (1.,1.5) ] # time in second
periodName = ['Pre-stim', 'Stim (Early)', 'Stim (Late)', 'Post-stim'];
freq = 40 # Hz
plt.figure(figsize=(16,3))
for periodIdx in range(len(period)):
tIdx = (EEG.times>period[periodIdx][0]) & (EEG.times<=period[periodIdx][1])
# Calculate power & Substitute bad-channel value
power = band_power(erp[:36,tIdx], np.array([-2,2])+freq, EEG.info['sfreq'])
power[bad_channels]= np.median(power.flatten())
# Draw
plt.subplot(1,len(period),periodIdx+1)
plot_topo2d(power, clim=(0,3) )
plt.title('%s, t = [%.1f, %.1f]s'%(periodName[periodIdx],period[periodIdx][0],period[periodIdx][1]))
plt.gcf().savefig(dir_fig+'fig3-3.png', format='png', dpi=300);
```
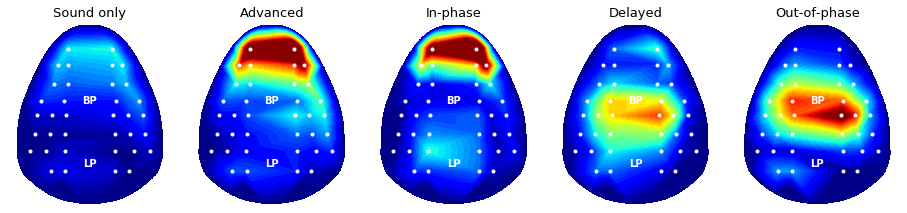
### 3-4. Band-power topography: Comparison across various experimental conditions
Applying the same routine above, power topography figures of five different experimental conditions can be drawn as below.
```python
# Demo 3-4. Band-power topography: Summary comparison across various stimulus conditions
freq = 40 # Hz
plt.figure(figsize=(16,4))
conditions = [6,4,1,3,2]
tIdx = (EEG.times>0) & (EEG.times<=1)
for targetCondition in conditions:
trialIdx = np.where((EEG.events[:,2])==targetCondition)[0]
erp = np.mean(EEG.data[:,:,trialIdx],2)
# Calculate power & Substitute bad-channel value
power = band_power(erp[:36,tIdx], np.array([-2,2])+freq, EEG.info['sfreq'])
power[bad_channels]= np.median(power.flatten())
# Draw
plt.subplot(1,len(conditions),np.where(np.array(conditions)==targetCondition)[0]+1)
plot_topo2d(power, clim=(0,7) )
plt.title('%s'%condNames[targetCondition-1], fontsize = font_size)
plt.axis('off')
if targetCondition is not conditions[0]: plt.ylabel('')
plt.subplots_adjust(wspace=.1, hspace=.3)
plt.gcf().savefig(dir_fig+'fig3-4.png', format='png', dpi=300);
```
Enjoy!
```python
# Try on your own!
```